Do Nitrogen and Iodine Form an Ionic Compound
While the other pairs sodium and potassium are the metals nitrogen and iodine. Iodine is in Group 17 of the Periodic Table.

Ionic Bonding Ca Standards Students Know Atoms Combine To Form Molecules By Sharing Electrons To Form Covalent O Ionic Bonding Covalent Bonding Ionic Radius
C chlorine and bromine.
. 2 Cl ions have a charge of 2 1 2Is potassium iodide an ionic or covalent compound. To form an ionic compound we need to have an overall charge that is zero sodium which is an alkali metal has one valence electron that it can donate Nitrogen needs three valence electrons to achieve an octet. Then identify the anion and write down its symbol and charge.
From the subscripts the compound contains one copper ion and two chloride ions. What is chemical bond ionic bond Molecular bond. That means it is a nonmetal.
Does a metal and non metal make a Ionic Bond Covalent Bond or both. 1 CsI Caesium generally forms Cs ions. Nitrogen and iodine helium and oxygen chlorine and bromine sodium and potassium.
See the answer See the answer done loading. Does Oxygen and iodine form a ionic compound. Do not form ionic bonds.
So when the metal and the non-metal make music together they form a ne. The first step is to prepare the NI 3One method is to simply pour up to a gram of iodine crystals into a a small volume of concentrated aqueous ammonia allow the contents to sit for 5 minutes then pour the liquid over a filter paper to collect the NI 3 which will be a dark brownblack solidHowever if you grind the. If the metal in the ionic compound is a transition element with variable charge metal ions the ionic.
That means it is a nonmetal. Does nitrogen and iodine form an ionic compound. Forms metallic bond.
Secondly does bromine and rubidium form an ionic compound. So when the metal and the non-metal make music together they form a ne. See the answer See the answer done loading.
The bond between sodium Will nitrogen and hydrogen form an ionic compound with. 6- lithium and calcuim. Your feedback makes Microsoft Bing a better search engine See morePNG See all imagesIonic compoundChemical CompoundChemical structureFunctionMolar massSolubility In chemistry an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding.
The key thing to look at here is the electronegativity difference in the two elements. Iodine is in Group 17 of the Periodic Table. A sodium atom transfers an electron to a chlorine atom to form a sodium ion and a chloride ion.
The first step is to prepare the NI 3One method is to simply pour up to a gram of iodine crystals into a a small volume of concentrated aqueous ammonia allow the contents to sit for 5 minutes then pour the liquid over a filter paper to collect the NI 3 which will be a dark brownblack solidHowever if you grind thedoes Oxygen and iodine form a ionic compound. Nitrogen triiodide is an inorganic compound with the formula NI3. Both elements are non-metals they do not form an ionic bond.
NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. Want to see the full answer. Nitrogen and oxygen are both nonmetals which almost always indicates the bond will be not be considered ionic.
From the subscripts the compound contains one copper ion and two chloride ions. The copper atom is positively charged and the iodine is negatively charged so there is an ionic bond between them. View the full answer.
Nitrogen is an element in group 5 of the periodic table. What is chemical bond ionic bond Molecular bond. How do you find the formula of an ionic compound To find the formula of an ionic compound first identify the cation and write down its symbol and charge.
Give the name of the ionic compound with the formula CuCl 2. F nitrogen and oxygen. The two elements are metals they do not form an ionic bond.
They do not form ionic bond. 6- lithium and calcuim. Does a metal and non metal make a Ionic Bond Covalent Bond or both.
The charge on both the cation and anion is same and is thus balanced. As such it will form an electron by losing a single electron to form a 1 ion. Iodine generally forms I ions.
It is an ionic compound. Both elements are non-metals they do not form an ionic bond. How to Perform the Nitrogen Triiodide Demo.
It can even be detonated by alpha radiation. Determine the chemical formula for the ionic compound that forms between cesium and iodine. Check out a sample QA here.
Potassium iodide is an ionic compound. Write the correct ionic formula for the compound formed between the following. Nitrogen and oxygen are both nonmetals which almost always indicates the bond will be not be considered ionic.
Potassium is in Group 1 of the Periodic Table. It is an ionic compound. Potassium iodide is an ionic compound.
It is an extremely sensitive contact explosive. Check out a sample QA here. So they can easily form ionic compound by the complete transfer of electrons.
5- nitrogen and iodine. Sodium and chlorine bond to form sodium chloride Which type of compound is sodium chloride. Does Oxygen and iodine form a ionic compound.
Hydrogen iodide HI is an ionic bond. 2 Cl ions have a charge of 2 1 2. Small quantities explode with a loud sharp snap when touched even lightly releasing a purple cloud of iodine vapor.
Related searches for Do Nitrogen and Iodine Form an Ionic Componitrogen iodine compoundnitrogen and iodine molecular compoundnitrogen and iodine formulanitrogen and iodine bondnitrogen compounds listcompounds that contain iodineiodine compound nameiodine compounds listPagination12345Next Not satisfied Very satisfied NextDo you want to tell us more. N has an electronegativity value of 30 and H has a value of 22. Do nitrogen and iodine form an ionic compound.
CThe compound formed between rubidium and bromine is an ionic compound because rubidium is a metal and bromine is a non-metal. Chlorine is in group 7A has 7 valency electrons and gains 1 8-71 to form Cl ions. E sodium and potassium.
D potassium and sulfur. A helium and oxygen. Which pair of elements is least likely to form an ionic compound.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic. Determine the chemical formula for the. The copper atom is positively charged and the iodine is negatively charged so there is an ionic bond between them.
Does Oxygen and iodine form a ionic compound. So they can easily form ionic compound by the complete transfer of electrons. Which pair of elements is least likely to form an ionic compound.
E sodium and potassium. While the other pairs sodium and potassium are the metals nitrogen and iodine chlorine and bromine helium and oxygen are the non-metals. Does iodine and nitrogen form an ionic compound.
Hydrogen iodide HI is an ionic bond. The bond between sodium. A helium and oxygen.
1 CsI Caesium generally forms Cs ions. Individual ions within an ionic compound usually have multiple nearest neighbours so are not considered to be part of molecules but instead part of a continuous three-dimensional network. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic Does sodium and nitrogen form an ionic compound.
I believe the answer is d and f. B magnesium and chlorine. NOT these four at bottom.
If you look at the Periodic Table of elements you will see that Nitrogen as well as iodine are nonmetals therfore. 5- nitrogen and iodine. CuI is an ionic compound that has each molecule made from one atom of copper Cu and one atom of iodine I.
The key thing to look at here is the electronegativity difference in the two elements. So they can easily form ionic compound by the complete transfer of electrons. Sulfur Nitrogen Iodine or Phosphorus.
Sodium and magnesium. Does iodine and nitrogen form an ionic compound. Nitrogen and iodine helium and oxygen chlorine and bromine sodium and potassium.
To form an ionic compound we need to have an overall charge that is zero sodium which is an alkali metal has one valence electron that it can donate Nitrogen needs three valence electrons to achieve an octet. That means it is a metal. NOT these four at bottom.
Chlorine is in group 7A has 7 valency electrons and gains 1 8-71 to form Cl ions. Iodine generally forms I ions. CThe compound formed between rubidium and bromine is an ionic compound because rubidium is a metal and bromine is a non-metal.
Expert AnswerHow do we name ionic compounds. It can even be detonated by alpha radiation. So when sodium and nitrogen get together to form an ionic compound Each sodium donates one valence electron to nitrogen so each sodium obtains a one plus charge.
No it is not Ionic. That means it is a metal. C chlorine and bromine.
Determine the chemical formula for the ionic compound that forms between cesium and iodine. Sodium is an element in group 1 of the periodic table. These can be simple ions such as the sodium and chloride in sodium chloride or polyatomic species such as the ammonium and carbonate ions in ammonium carbonate.
The subscript for both calcium and oxygen is 1. Ionic compounds usually form crystalline structures when solidWikipedia People also search forIonic BondingCovalent BondPolyatomic IonIonLewis StructureSee all 5Data from. The two elements are metals they do not form an ionic bond.
The charge on both the cation and anion is same and is thus balanced. Is nitrogen dioxide ionic or covalent. Do not form ionic bonds.
Small quantities explode with a loud sharp snap when touched even lightly releasing a purple cloud of iodine vapor. Potassium is in Group 1 of the Periodic Table. Finally combine the two ions to form an electrically neutral compound.
Sulfur Nitrogen Iodine or Phosphorus. How do ionic compounds form from sodium and nitrogen. When it forms the nitride ion it gains three electrons to form a 3- ion.
Metals react with nonmetals to form ionic compounds. Sodium and chlorine bond to form sodium chloride Which type of compound is sodium chloride. View the full answer.
Want to see the full answer. From the given pairs of elements potassium and sulfur magnesium and chlorine are form ionic compound because potassium and magnesium are the metals and sulfur and chlorine are the non-metals. Which of the following pairs of elements are likely to form ionic compounds.
Give the name of the ionic compound with the formula CuCl 2. Ionic bond is a chemical bond formed by the complete transfer of electrons between two atoms. Sodium and magnesium.
The subscript for both calcium and oxygen is 1Solved Please help. I believe the answer is d and f. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.
An ionic compound is a metal and a nonmetal mixed together. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds. By signing up youll get thousands of step-by-step solutions to your homework.
F nitrogen and oxygen. It is an extremely sensitive contact explosive. Write the correct ionic formula for the compound formed between the following.
Which of the following pairs of elements are likely to form ionic compounds. B magnesium and chlorine. Does Oxygen and iodine form a ionic compound.
Total charge on one formula unit 3 3 0. Metals react with nonmetals to form ionic compounds. The compound is neutral overall but consists of positively charged ions called cations and negatively charged ions called anions.
D potassium and sulfur. Furthermore Is na2cl a.
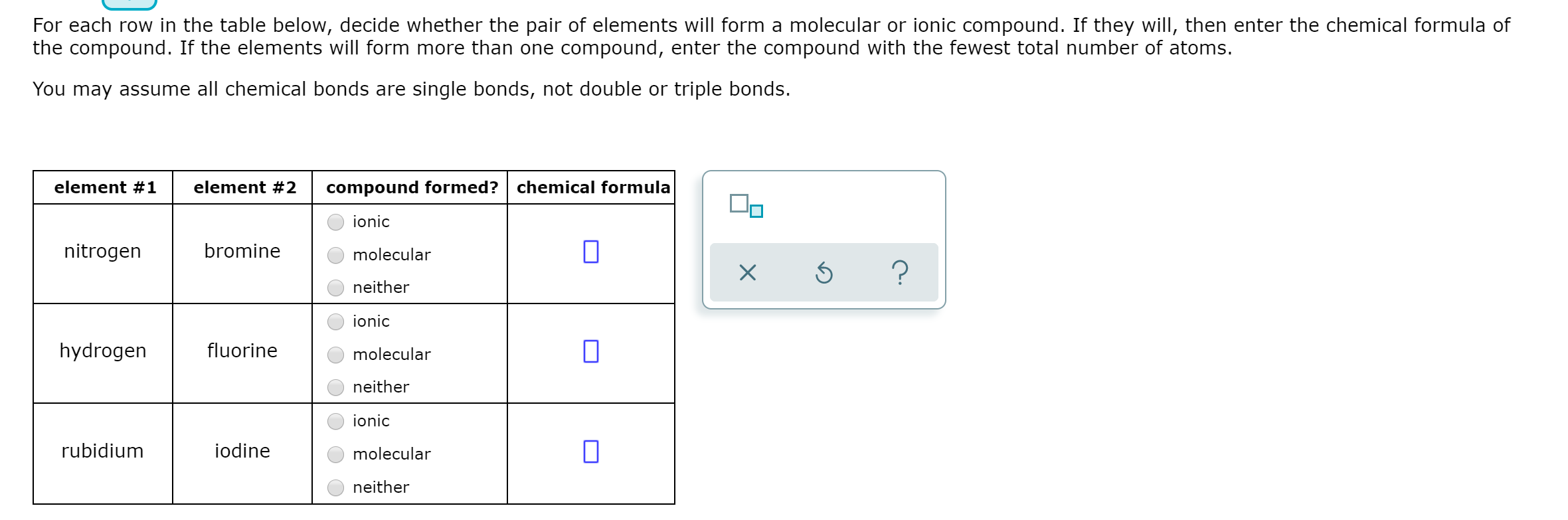
Solved For Each Row In The Table Below Decide Whether The Chegg Com
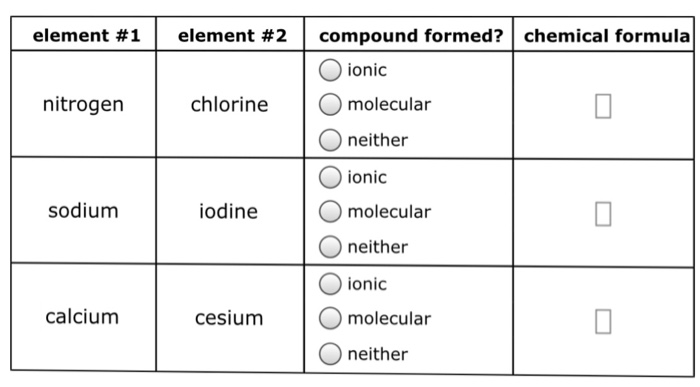
Solved Element 1 Element 2 Compound Formed Chemical Chegg Com
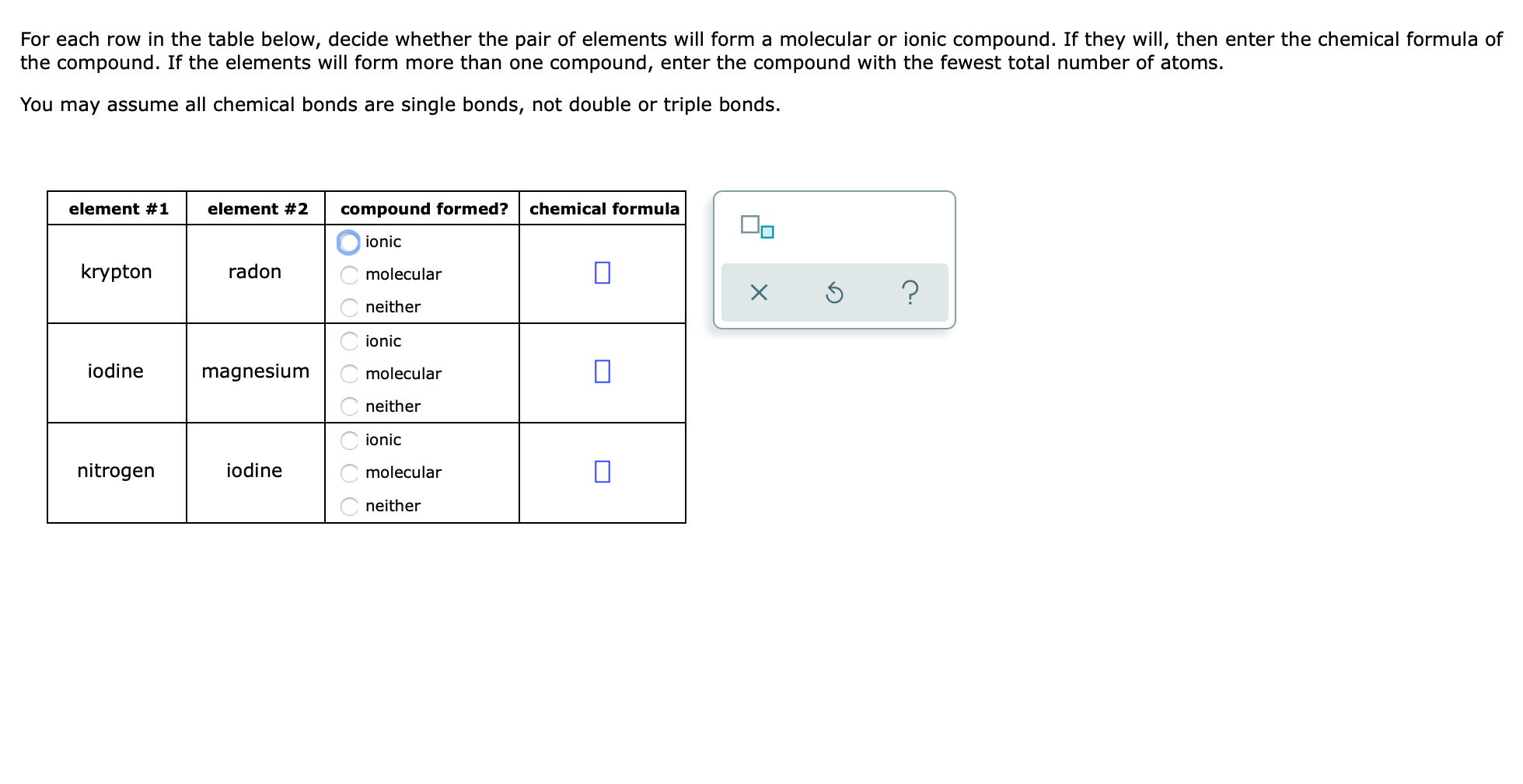
Solved For Each Row In The Table Below Decide Whether The Chegg Com
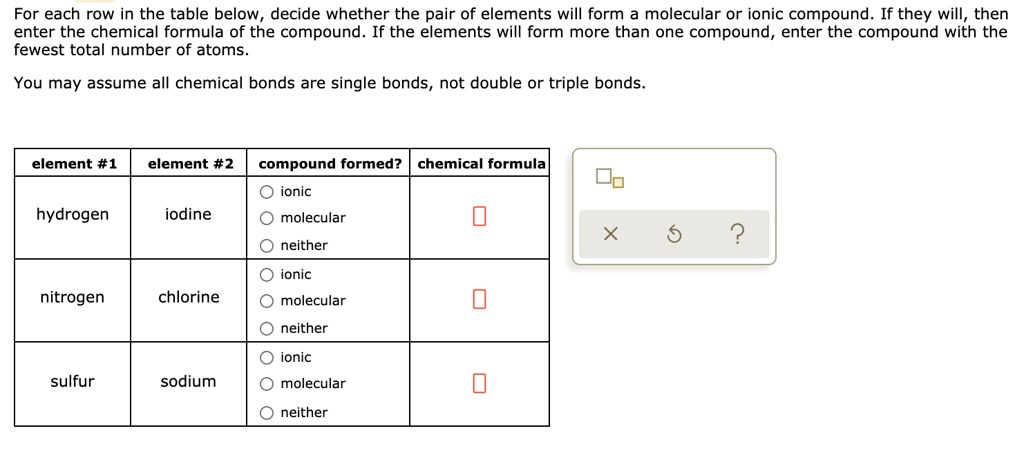
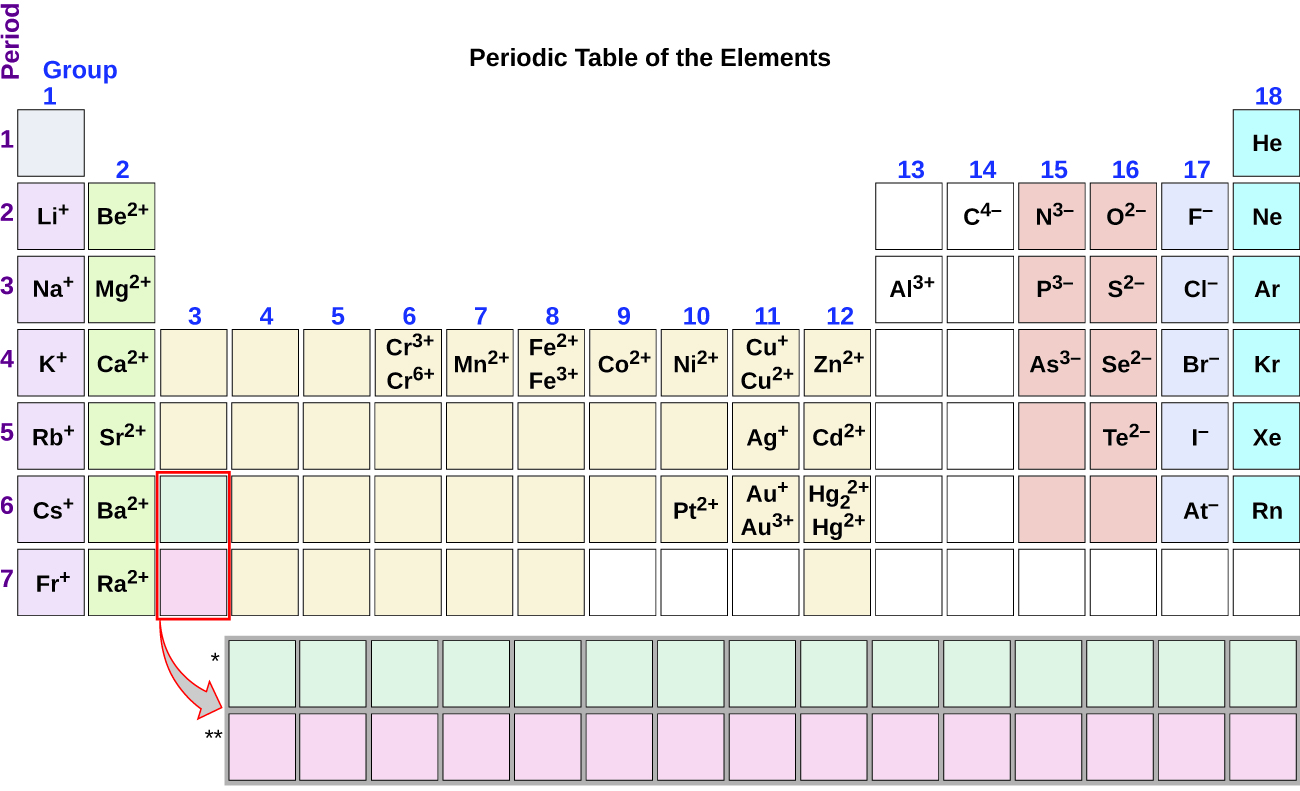
Solved For Each Row In The Table Below Decide Whether The Pair Of Elements Will Form A Molecular Or Ionic Compound If They Will Then Enter The Chemical Formula Of The Compound If

No2 Lewis Structure Nitrogen Dioxide Nitrogen Dioxide Molecules Lewis

Image Result For Naming Binary Ionic Compounds Ionic Compound Chemistry Worksheets Chemistry Lessons

Solved Which Of The Following Pairs Of Elements Are Likely To Form Ionic Compounds Check All That Brainly Com
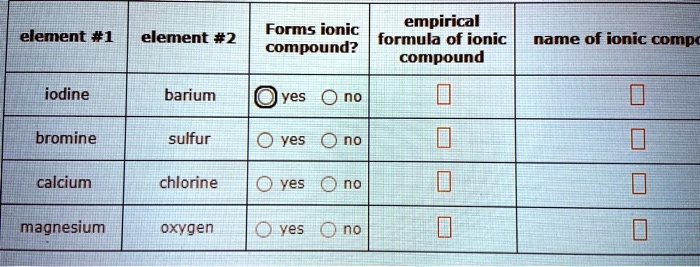
Solved Forms Ionic Empirical Formula Of Ionic Compound Compound Element 1 Element 2 Name Of Ionic Comp Iodine Barium Yes Ono Bromine Sulfur Yes Ono Calcium Chlorine O Yes Ono Magnesium Oxygen Yes
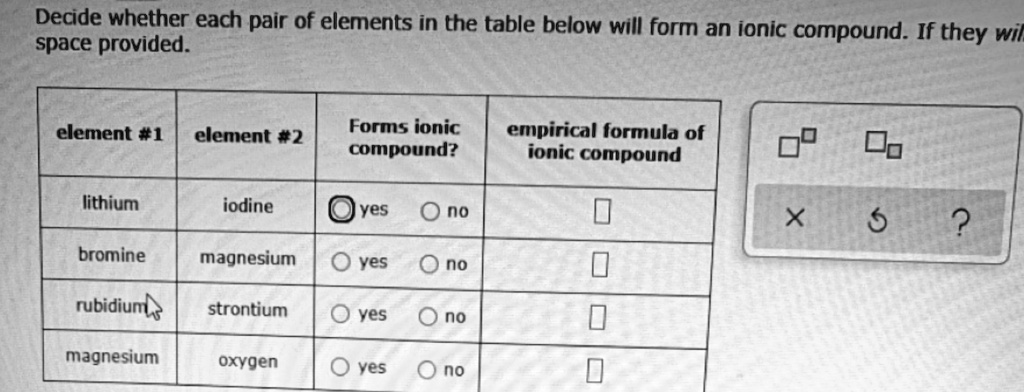
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If Space Provided They Wil Element 1 Element 2 Forms Ionic Compound Empirical Iormula Of Ionic Compound

Ionic Compounds Writing Formulas Ppt Video Online Download

2 6 Molecular And Ionic Compounds Chemistry
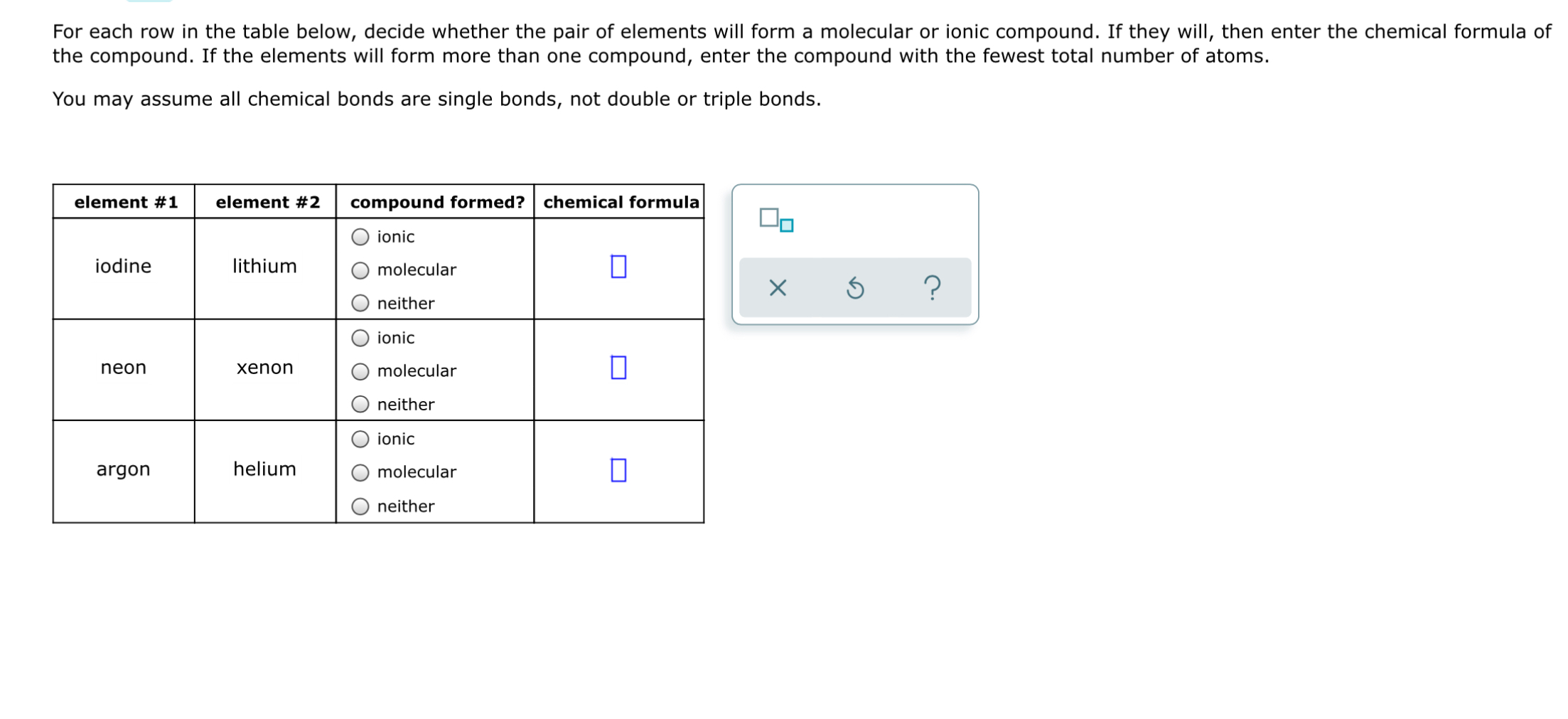
Solved For Each Row In The Table Below Decide Whether The Chegg Com
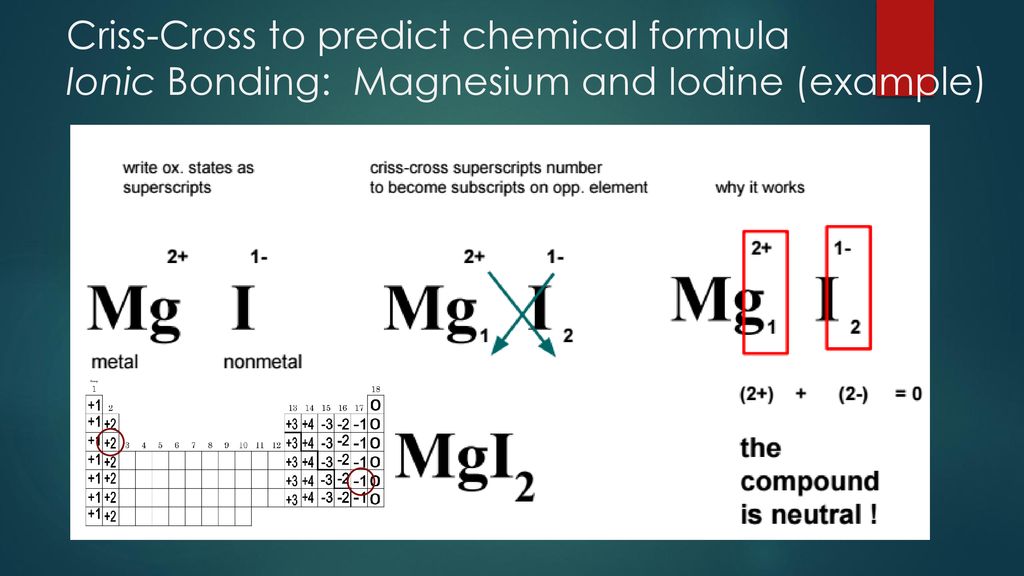
Bonding Ionic Bonding Ppt Download

Section 6 3 Ionic Bonding And Ionic Compounds Ppt Download

Solved Name The Ionic Compound Formed From Cach Of The Following Pairs Of A Magnesium Und Nitrogen B Iodine Und Cadmium C Strontium And Fluorine Sulfur And Cesium Name The Following Anions

Ionic Bond Examples Biology Dictionary

Lewis Structure Of Csi Caesium Iodide Youtube
Solved Determine Whether The Following Pairs Of Elements Can Chegg Com
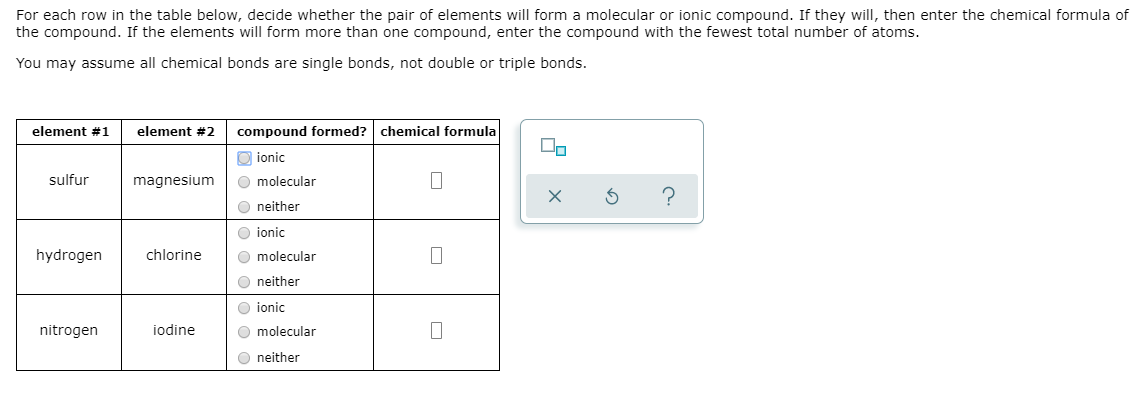
Solved For Each Row In The Table Below Decide Whether The Chegg Com
Comments
Post a Comment